1. Prepare the wound and surrounding skin according to local clinical practice.
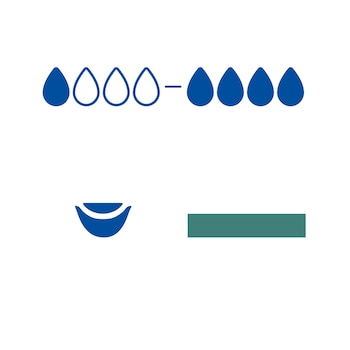
2. Select an appropriate dressing size for the wound. The dressing can be unfolded into suitable size and may overlap the wound margins if needed.
3. Remove the dressing from the peel pouch using an aseptic technique.
4. If the dressing is cut, use an aseptic technique. Do not tear the dressing. Discard any unused dressing.
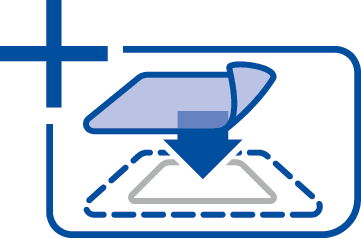
5. Apply the dressing. Ensure that the dressing comes into direct contact with the complete wound surface to allow microorganisms to bind to the dressing. In deep wounds, unfold and fluff up one or more dressings and fill the wound. Avoid dense packing. The dressing can also be used as a liner.
6. Apply a secondary dressing appropriate for the exudate level such as a foam dressing, absorbent pad or gelling fiber dressing, and fixate.
7. The dressing change frequency depends on exudate levels and the overall condition of the wound and surrounding skin. Should the clinical condition allow, the dressing can be left in place for up to 7 days.
How to removeShould the dressing adhere to the wound, moisten the dressing to assist removal and to avoid disruption of the healing wound.
For detailed product information, including indications for use, contraindications, precautions and warnings, please consult the product’s Instructions for Use (IFU).